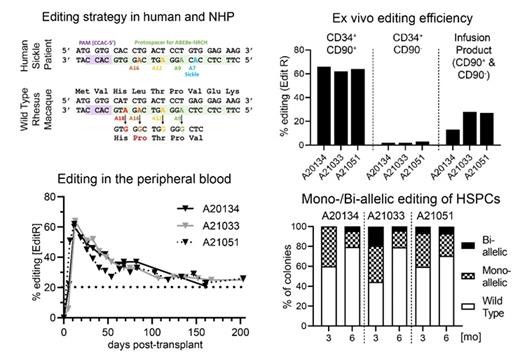
Sickle cell disease (SCD) is caused by a single nucleotide change in the β-globin chain. This nucleotide can be modified using adenine base editors (ABEs) to convert the defective SCD β-globin gene (HBB S) into the non-pathogenic Makassar β-globin (HBB M) variant. Proof-of-concept for this editing strategy in human and mouse hematopoietic stem cells (HSCs) has been previously demonstrated by transplantation after ex vivo modification as well as by in vivo adenoviral editing followed by selection. Here, we evaluated the feasibility, long-term efficiency, and safety of autologous ex vivo Makassar base editing in the preclinical nonhuman primate (NHP) large animal model to pave the way towards clinical applications in humans. As NHPs don't carry the sickle mutation (position A7 in the protospacer), bystander editing at position A9 was monitored as a surrogate.
NHP CD34 + hematopoietic stem and progenitor cells (HSPCs) were enriched, CD34 +CD90 + HSCs FACS-purified as previously reported to increase the editing efficiency of short- and long-term engrafting cells, primed over-night, and ABE8e-NRCH base editor mRNA was delivered by electroporation. Base-edited HSCs were cultured overnight and then combined with unmodified CD34 +CD90 - cells and infused into animals after myeloablative total body irradiation. The infusion product was quality controlled by flow-cytometry, next generation sequencing (NGS), and assessing the mono-/bi-allelic base editing efficiency on a single cell level from colony-forming cell (CFC) assays. Animals were followed for up to 200 days analyzing the editing efficiency in peripheral blood (PB) white blood cells (WBCs), FACS-purified lineages from the PB, bone marrow (BM) WBCs, as well as FACS-purified BM HSPCs. Comprehensive bioinformatics analysis was performed on the generated NGS data to determine the frequency of editing over time as well as the on- and off-target editing within the protospacer at all four target locations. Finally, chromosomal integrity after base editing was confirmed performing Kromatid karyotyping assays on gene-edited BM-derived HSPCs.
Ex vivo base editing of CD34 +CD90 + was highly reproducible at the Makassar locus reaching more than 60% efficiency within bulk HSCs. No impact of base editing on the myeloid and erythroid differentiation of HSCs was seen in CFC assays. Clonal analysis of colonies revealed that more than 90% of HSCs in the infusion product were edited with > 20% of cells showing biallelic editing. Gene-edited HSCs combined with unmodified progenitors rapidly engrafted in myeloablated animals without any noticeable delay in the recovery of neutrophils, red blood cells, as well as platelets and multilineage reconstitution was established within 3-6 months. Most importantly, stable editing of >20% was observed in PB WBCs and across all lineages throughout the entire follow-up. Similarly, the BM stem cell compartment fully recovered to baseline within 6-month post-transplant. Engrafted HSPCs demonstrated normal erythro-myeloid colony-formation potential. Assessment of editing in colonies showed persistence of HSCs with mono- and bi-allelic Makassar editing 6-month post-transplant. Closely matching the editing efficiency in the PB, all BM lineages, HSPC subsets, and colonies demonstrated >20% editing efficiency confirming successful editing of long-term persisting multipotent HSCs. Finally, comprehensive assessment of NGS data from all tissues in conjunction with the Kromatid assay revealed no unwanted off-target effects or chromosomal rearrangements. Editing was primarily observed at positions A9 and A12 confirming highly specific on-target editing.
In summary, we demonstrate efficient, persistent, and safe base editing of NHP HSCs for the treatment of SCD. The editing efficiency of the bystander adenine in NHP HSCs is almost identical to the previously observed editing in human patient HSCs to generate the Makassar HBB. Base edited HSCs showed no impairment in homing, long-term engraftment, or multilineage differentiation in the NHP. These findings have major implications for the application of base editing in patients with hematological disease and disorders.
Disclosures
Radtke:Proteios: Consultancy, Current equity holder in private company, Membership on an entity's Board of Directors or advisory committees; 48 Bio: Consultancy; Ensoma: Consultancy. Yen:Beam therapeutics: Current equity holder in publicly-traded company; IMAGO/Merck: Consultancy. Weiss:Novartis Inc.: Consultancy; Vertex Pharmaceuticals: Consultancy; Cellarity: Current equity holder in private company; bluebird bio: Consultancy; GlaxoSmithKline: Consultancy. Kiem:Homology Medicines: Consultancy; Rocket Pharmaceuticals: Consultancy; Ensoma: Consultancy; V.O.R. Biopharma: Consultancy; CSL Behring: Consultancy; Magenta Therapeutics: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal